Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Contents
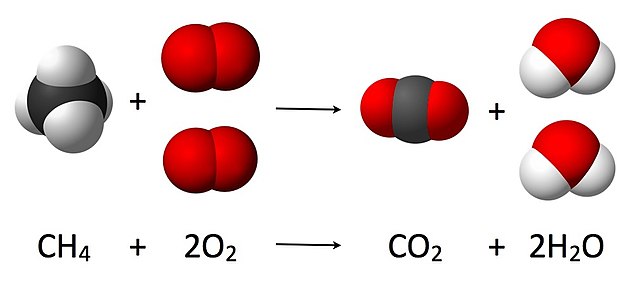
The Law of Conservation of Mass, discovered by Antoine Lavoisier in 1789, states that mass remains constant in a closed system during a chemical reaction. This means that the total mass of all reactants and products in a chemical reaction remains the same at any given point in time. The law revolutionized the field of chemistry and laid the foundation for modern scientific principles.
Elements on Earth are stable, and their mass remains constant during chemical reactions. Atoms that make up living and nonliving matter have a long history, cycling through various compounds without being created or destroyed. Ecologists use the law of conservation of mass to analyze elemental cycles and understand the flow of matter in ecosystems.
Ecosystems can be viewed as a battleground for essential elements like oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. Living organisms are primarily composed of these elements, and their cycling through the Earth system is crucial for ecosystem functioning. The law of conservation of mass ensures that the total mass of elements in an ecosystem remains constant over time.
For example, in forests, the mass balance of elements plays a vital role. Early successional forests gain biomass as trees grow, acting as carbon sinks. In mature forests, the carbon taken up through photosynthesis equals the carbon respired by the ecosystem, maintaining a steady carbon balance. When forests are cut down, the stored carbon is released back into the atmosphere, emphasizing the importance of mass balance in understanding ecosystem dynamics.
Individual organisms also follow the law of conservation of mass. Each organism has a unique elemental composition determined by its form and function. The availability of essential elements influences an organism’s health and reproduction. Failure to meet elemental demands can lead to poor health, limited reproduction, and even extinction, as seen in the case of the Irish Elk.
Organisms continuously replace their chemical makeup by incorporating necessary elements and releasing waste products. The recycling of elements after an organism’s death is essential for sustaining life on Earth. Understanding the mass balance in organisms helps in studying their nutritional requirements and ecological roles.
The law of conservation of mass applies not only to natural ecosystems but also to human-dominated systems like cities and agricultural fields. Cities import materials like food, fuel, and water, and export waste products. Agricultural systems, both traditional and modern, can be analyzed using mass balance principles to optimize resource use and minimize environmental impact.
As the global population grows, the challenge of maintaining mass balance in human systems becomes more critical. Efficient resource management and sustainable practices are essential to ensure the long-term viability of human-dominated ecosystems. The law of conservation of mass serves as a guiding principle in addressing the complex interactions between human activities and the environment.
The Law of Conservation of Mass is a fundamental principle in chemistry and ecology, stating that mass remains constant in a closed system. From chemical reactions to ecosystem dynamics, the concept of mass balance plays a crucial role in understanding the flow of matter in various systems. By applying this law, scientists can unravel the intricate relationships between elements, organisms, and ecosystems, paving the way for sustainable practices and environmental conservation.